Is Temperature Inversely Proportional to Pressure
Asked Feb 17 2019 in Trades Technology by david Answer the following statement true T or false F. This relationship between pressure and volume is known as Boyles law after its discoverer and can be stated as follows.

Boyle S Law Boyle S Law Law Chemistry
The relationship between pressure and temperature of a gas is stated by Gay-Lussacs pressure temperature law.

. Combining the above and the Avagadros law we get the. Meaning Pressure is inversely proportional to Volume or or where P is the pressure and V is the. Boyles law states that pressure P and volume V are inversely proportional.
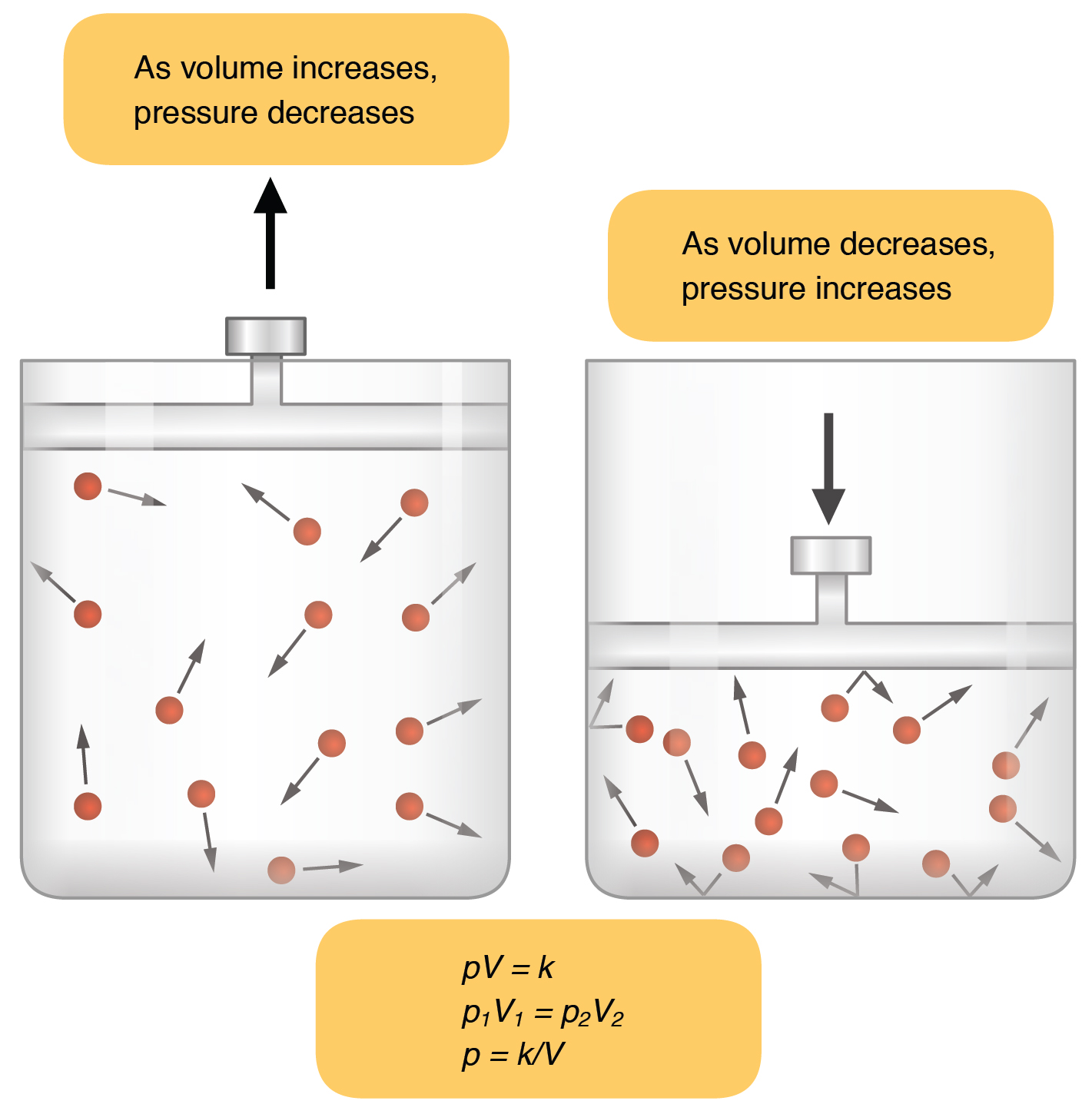
According to Boyles law at constant temperature pressure is inversely proportional to volume. Answer 1 of 7. Is heat directly proportional to temperature.
Therefore as the pressure of a particular system goes up the temperature of that system also goes up and. We find that temperature and pressure are linearly related and if the temperature is on the kelvin scale then P and T are directly proportional again when volume and moles of gas are held constant. Now we are given that At a pressure of 30 pounds per square inch psi a gas has a volume of 600 cubic inches.
Pressure and temperature are not inversely proportional. At constant temperature the volume of a fixed amount of a gas is inversely proportional to its pressure. Let the pressure be x.
Since we are given that the volume of the gas is inversely proportional to its pressure. Charles law states that volume V and temperature T. If volume and pressure are both directly proportional to temperature when the other is held constant combination of Gay-Lussacs Law and Charles Law you can write the proportionality as P V T.
Let the proportionality be k. Temperature is directly proportional to pressure2Pressure is inversely proportional to VolumeBut in summers let us say at 45 C if we fill air in tyres of bikes as the temperature is high pressure should be high and when pressure becomes high volume should decreaseBut this does not happenWhy. In fact if the volume is held constant temperature and pressure are directly proportional to each other.
Pressure is inversely proportional to volume when the temperature is held constant for a given amount of gas. Temperature and pressure are not inversely proportional. Y 600.
Pressure and temperature are inversely proportional to each other. If the temperature on the kelvin scale increases by a certain factor the gas pressure increases by the same factor. All of the above none of the above b.
For a given mass of an ideal gas the volume and amount moles of the gas are directly proportional if the temperature and pressure are constant. B The unit of measurement for pressure preferred by many respiratory therapists is ANSWER. Substitute the value of k in A.
One way to think about it is to. Inversely proportional to temperature. Higher the pressure so is the temperature and vice versa.
Both directly proportional to pressure and directly proportional to temperature. This law states that the pressure P of a fixed mass of gas held at a constant volume is directionally proportional to its Kelvin temperature T. Relationship Between Altitude And Air Pressure Amazing Answer 2022FAQWhat The Relationship Between Altitude And Air Pressure Amazing Answer 2022adminSend emailNovember 24 2021 minutes read You are watching What The Relationship Between Altitude And Air Pressure.
If volume and pressure are inversely proportional at constant temperature Boyles Law then you can write the proportionality as P 1 V. It is also known as Boyles lawPressure is directly proportional to the temperature when the volume is held constant for a given amount of gas. It is also known as Gay-Lussac law.
According to Charles law at constant pressure volume is directly proportional to temperature. Directly proportional to pressure. Avogadros law hypothesized in 1811 states that at a constant temperature and pressure the volume occupied by an ideal gas is directly proportional to the number of molecules of the gas present in the container.
The ideal gas equation gives us PVnRT P is directly proportional to T. So substitute x 30.

Boyle S Law Gas Law State Of Matter Chimica
Scientific Accomplishments Robert Boyle Is Most Known For His Creation Of Boyle S Law In Chemistry Which St Chemistry Classroom Chemistry Lessons Boyle S Law

Pin On Best Chemistry Calculator

Henrys Law Easy Science Easy Science Chemistry Experiments 10th Grade Science

Gas Laws Of Respiratory Physiology Boyle S The Pressure Of A Given Quantity Of Gas Is Inversely Proportional To Physiology Dalton S Law Anatomy And Physiology

Gas Properties Teaching Chemistry Thermodynamics Gas

Pin Na Doske Chemistry Assignment

Boyle S Law The Volume Of A Fixed Quantity Of Gas Maintained At Constant Temperature Is Inversely Proportional To The Pressure Can Be Represented Mathematicall

Pin On Mechanical Engineering Basics

What Are The Properties Of Boyle S Law Socratic Boyle S Law Law Software Update

Boyle S Law Boyle S Law States That The Relationship Between The Pressure And Volume Of Gases Is Inversely Propor Boyle S Law Pulmonary Gas Exchange Physiology

The Gas Laws Science Educational School Posters Chemistry Classroom Chemistry Lessons Teaching Chemistry

The Gas Laws Science Educational School Posters School Posters Education Emergency Nursing

Pin By Joel Broughton On Nursing Education Chemistry Lessons Physical Science High School Study Chemistry

Gas Laws Boyle S Law Study Chemistry Chemistry Worksheets

Use Boyle S Law To Find The Volume Of A Gas Balloons What S My Favorite Color Bubble Balloons



Comments
Post a Comment